| HOME |
| LEADERSHIP |
| SERVICES |
| PUBLIC SEMINARS |
| REGISTER |
| ARTICLES |
| SITE MAP |
| CONTACT |
| ESPAÑOL |
Installation Qualification (IQ)
Our facilities engineering group gives us unique IQ capabilities.
Operational Qualification - (OQ)
Process Control Limits
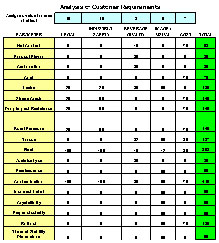
Effective process control depends on the statistically sound selection of:
- process and product parameters
- control charts
 sampling
plans
sampling
plans
- calculation method for control limits
- SOPs for responding to control signals.
Software Parameters
We represent, install, and train in the use of Infinity QS® SPC software. Medical Device and Pharmaceutical companies use Infinity QS® SPC software for all features necessary to meet FDA requirements.
Raw material specifications
DOE and SPC form the basis for establishing raw material specifications and quality requirements.
Process Operating Procedures
Process engineers use DOE to develop process models and "Quick Guides" for confidently managing the process.
Material Handling Requirements
DOE and SPC help you identify critical handing parameters and SOPs necessary to ensure the quality of the final product.
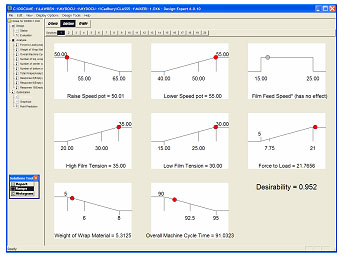
Process Change Control
The use of the "Quick Guides" and SOPs help with the reconfirmation of process capability and assure smooth, safe process modification when required.
Training
We have been assisting operations with process improvement, efficiency improvement, and training and implementing Six Sigma methodologies for 15 years. Our staff has over 25 years of experience as trainers and practitioners of SPC/SQC and DOE.
Short term stability and capability of the process, (latitude studies or control charts)
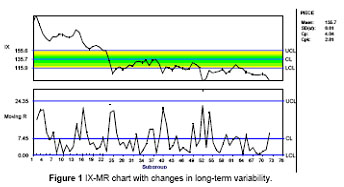 As
practitioners of SPC/SQC/DOE and Six Sigma methodologies, we routinely assist
clients with obtaining and maintaining stable processes capable of meeting
customer, consumer requirements and government regulations. Cp, Cpk calculations
indicate short term process capability.
As
practitioners of SPC/SQC/DOE and Six Sigma methodologies, we routinely assist
clients with obtaining and maintaining stable processes capable of meeting
customer, consumer requirements and government regulations. Cp, Cpk calculations
indicate short term process capability.
Potential Failure Modes, Action Levels and Worst-case Conditions (Failure Mode and Effects Analysis, Fault Tree Analysis)
FMEA and DOE anticipate potential failure modes. DOE allows you to model processes enabling "what if" scenarios to understand the effects of changes in raw material, ambient conditions, changes in the process, etc., have on the process. You can avoid potential failures using the process models, SPC, "Quick Guides" and SOPs. DOE also assists in understanding the process and product and helps guide redesigns that result in more robust processes and products.
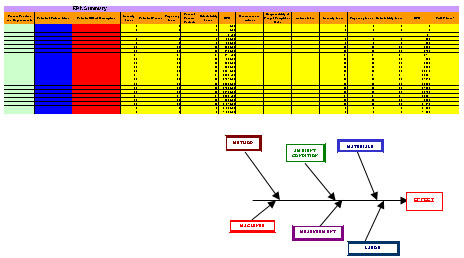
Screening Experiments
Establish key process parameters and statistically designed experiments help to optimize the process during this phase.
Sound statistical techniques establish the important process variables to control and how to control them. DOE and SPC aids you in optimizing processes and establishing guides and SOPs for controlling them to maintain optimum performance.
Performance Qualification - (PQ)
 Actual
Product and Process Parameters and Procedures Established in OQ
Actual
Product and Process Parameters and Procedures Established in OQ
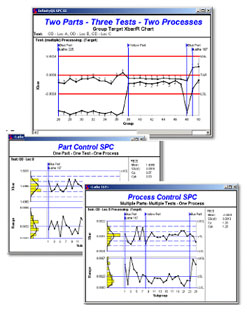
We train and implement SPC/SQC procedures, SOPs Quick Guides including control charting, reacting to out of control points and verifying measurement precision and accuracy. Product and process parameters measured, control limits, SOPs, and Quick Guides are developed during OQ.
Acceptability of the Product
We facilitate functional analysis to understand the important product and process parameters to measure and their appropriate acceptable levels necessary to meet all customer, consumer, government and legal
Assurance of Process Capability as established in OQ
We implement continuous monitoring of Pp and Ppk to understand if the process is performing to capability. If not, SOP's document corrective actions necessary.
Process Repeatability, Long Term Process Stability
We implement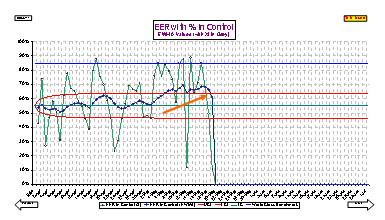 the use or EWMA charts to monitor on going process stability and
product quality.
the use or EWMA charts to monitor on going process stability and
product quality.
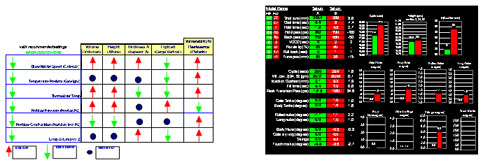
We use DOE during OQ and PQ to establish the key attributes for continuous monitoring and maintenance. Process and product data should is analyzed using control charts to identify and correct any variation due to controllable causes. Depending on the nature of the process and its sensitivity, controllable causes of variation may include:
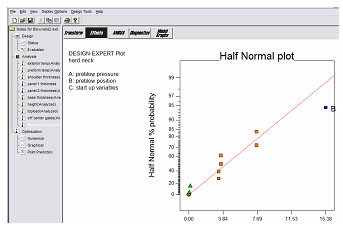 Temperature
Temperature
- Humidity
- Variations in electrical supply
- Vibration
- Environmental contaminants
- Purity of process water
- Light
- Human factors (training, ergonomic factors, stress, etc.)
- Variability of materials
- Wear and tear of equipment
We use DOE to identify the source of variation and to understand how to eliminate, control, or minimize its effect on the process and product.
Maintaining a State of Validation
Monitor and Control
We implement the use of SPC to monitor trends in the process to ensure the process remains within the established parameters. We train and implement the use of control charting (Xi/Xbar/Attribute control charts/EWMA charts) to monitor the process and provide an alarm for when the process or product quality may be headed toward an out of control condition. Should a trend towards or an out of control condition occur we implement SOPs and Quick Guides that outline specific investigative measures and corrective actions necessary. Our experienced experts are prepared to assist in any way necessary should revalidation be required.
Changes in Processes and/or Product
Should changes in the process and /or product including changes in procedures, equipment, personnel, etc. require revalidation, our expert staff is prepared to assist in any way necessary.
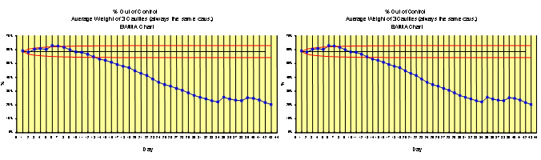
Continued State of Control
We conduct DOE's and develop mathematical process models to understand the effect of variability due to changes that may occur in raw materials and/or processes. These may be difficult to detect by conventional means or may be considered inconsequential incorrectly. (An example of this type of process is sterilization.) We implement SPC procedures, Quick Guides, and SOPs based on our DOE results and the process models to ensure that these changes are detected and corrective action taken. Our expert staff is prepared to assist if periodic revalidation is necessary.
Continuous Improvement
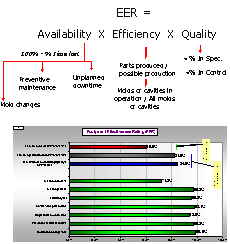
A key component of ZDM's programs is performance measurement. The key in effective performance is a system of measures that measure key strategic activities such as reduction of variation and the net effects of our efforts on productivity and quality. In ZDM's programs, we measure not only how much we produce but how much consistent quality product we produce and how well it meets the customer requirements.
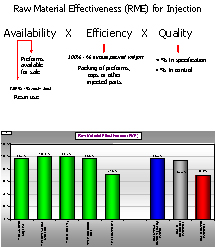
Typical strategic activities which we measure are total daily variation and the % of samples detected outside statistical process control limits. Equipment Effective Ratings (EER) and Raw Material Efficiency (RME) look at the effectiveness with which we use capital invested in equipment, space, energy, Raw materials and labor to produce consistent quality goods. Our EER and RME not only take into account conventional parameters of efficiency when running, unplanned downtime, planned maintenance, changeovers, etc., but also consistency, that is in control and in specification. Our certification method for operations utilizes EER and RME benchmarked against performance targets based on within organization or industry bench marks.